Explain Difference Between Atomic Mass and Atomic Number
It is the. It is denoted by symbol z.

Atomic Number And Mass Number Introduction To Chemistry
The atomic number is the number of electrons present in an atom.

. If an element X has a mass number of 92 and 40 protons please explain how you could obtain the number of neutrons and electrons of this element. A Z n. The number of protons in the nucleus of an atom Atomic mass.
It is represented by A. Atomic number refers to the number of protons in the nucleus of an atom. The mass number is the sum of the number of protons and neutrons in an atom.
3 rows Atomic number is used to define the type of element a material or substance is. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. When measuring the mass of an atom we actually measure the mass of the nucleus.
The mass number is a simplified number. Explain the difference between atomic mass and atomic number. Explain the difference between the atomic number and the atomic mass.
Then ii define the term isotope and iii state which values-mass number atomic number atomic mass-differs between isotopes. What is the difference between atomic number and mass number. 2 Get Other questions on the subject.
The atomic mass number is the number of protons and the number of neutrons. What is the difference between Mass Number and Atomic Mass. 5 rows The major difference between atomic number and mass number is that the atomic number.
Atoms are composed of electrons protons and neutronsProtons and neutrons together make the nucleus of an atom. It is a decimal number. Potassiums atomic number is 19 19 electrons and its atomic mass.
We know Atomic Number No. We often use the terms atomic mass and average atomic mass. The key difference between atomic mass and average atomic mass is that the atomic mass is the mass of an atom whereas the average atomic mass is the mass of an atom of a particular chemical element calculated by considering isotopes of that element.
Explain the difference between the MASS NUMBER and the ATOMIC NUMBER of a nuclide. October 7 2019 Posted by Madhu. Mass Number No.
Oct 31 2015. Explain the difference between atomic numbers and atomic mass. The atomic number deals with several decimal values as it is a weighted average.
Pallava BaglaGetty Images. Main Difference Atomic Number vs Mass Number. Relationship Between Mass Number and Atomic Number.
6 rows Difference Between Atomic Mass and Atomic Number. So the atomic mass refers to the mass than Adam in units of atomic mass units or the mass of a mole of atoms in units of grams it takes into account everything that is present. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.
AZ represents the number of neutrons. If an element X has a mass number of 92 and 40 protons please explain how you could obtain the number of neutrons and electrons. On the lines provided label the sections as metals nonmetals.
Atomic mass is the weighted average of all naturally occurring isotopes of. Therefore we can say. Mass Number Atomic Number No.
A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. MASS NUMBER is the total number of protons in the nucleus of each atom of an element. Atomic number is the number of protons in a nucleus of an atom.
The mass of an average atom of an element in atomic mass units. The protons the neutrons and the small mass associated with the electrons. It is equal to the number of protons in a neutral atom.
Atomic mass when expressed in amu is the sum of the number of protons and the number of neutrons. ATOMIC NUMBER Is the total number of protons in the nucleus of an isotope. That is because the mass of an electron is negligible when compared to a proton or a neutron.
It is a whole number. 1 Explain the difference between mass number atomic number and atomic mass. Usually without decimal places.
The atomic number is the number of electrons in an atom and the atomic mass is the sum of electrons and neutrons in a atom. The mass number also called atomic mass number or nucleon number is the total number of protons and neutrons in an atomic nucleus. The Relationship Between Mass Number and Atomic Number is stated as.
1 Explain the difference between mass number atomic number and atomic mass. Electrons contribute so little mass that they arent counted. Mass number is the total number of protons and neutrons in the nucleus.
Fill in the table Subatomic Particle Charge Mass Proton positive 1 amu Electron - Negative 11800 amu Neutron No chargeneutral 1 amu 29. Of protons No. Consider 39K a The mass number A is 39 b The atomic number Z is 39 c Blank d The atomic number Z is 19 e The mass number A is 20 arrow_forward Copper has 2 stable isotopes namely Cu-63 and Cu-65.
Mass number is the number of protons neutrons in one particular atom. Atomic mass m a is the mass of an atom. The density of helium in the blimp is 1786 kilogrammeter3.
Of helium in a blimp is 628 x 109 millimeters. Symbolically this can be represented by. Conventionally atomic number is written in the left bottom corner of an element whereas the mass number is written in the left upper corner.
Difference Between Atomic Number And Atomic Weight Definition Explanation Difference
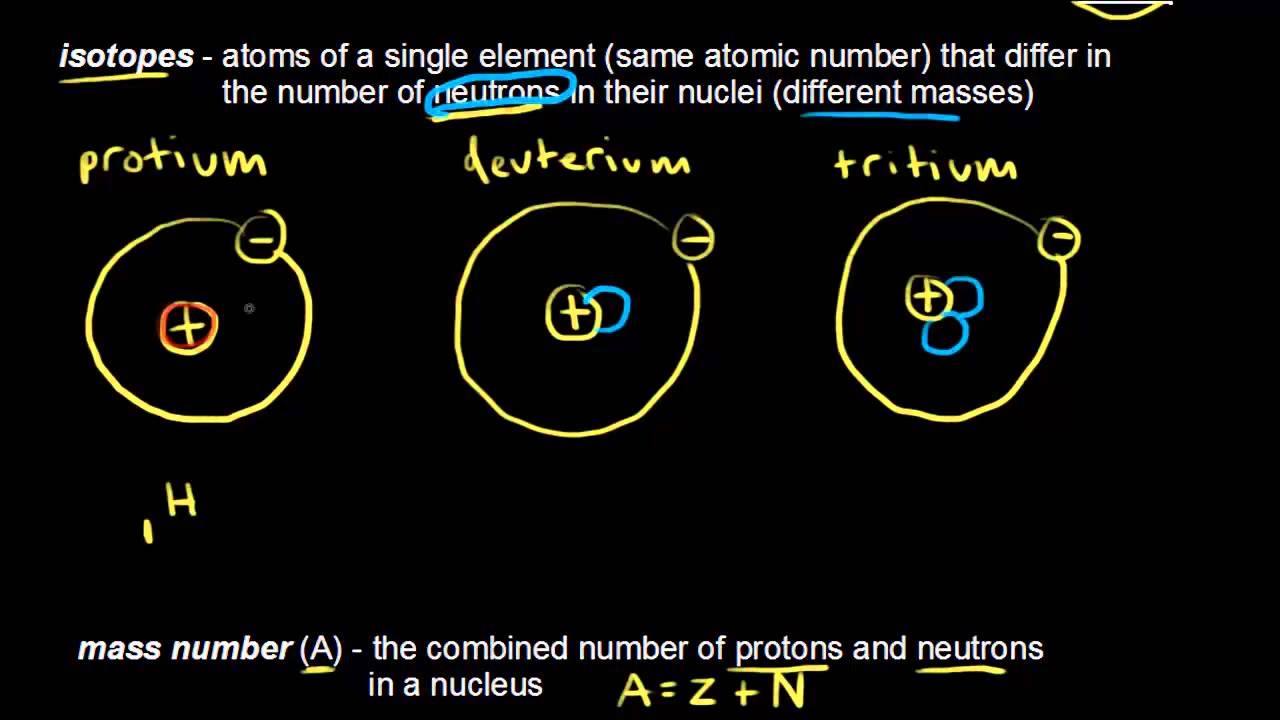
Atomic Number Mass Number And Isotopes Video Khan Academy
Difference Between Atomic Mass And Atomic Weight Atomic Mass Vs Atomic Weight
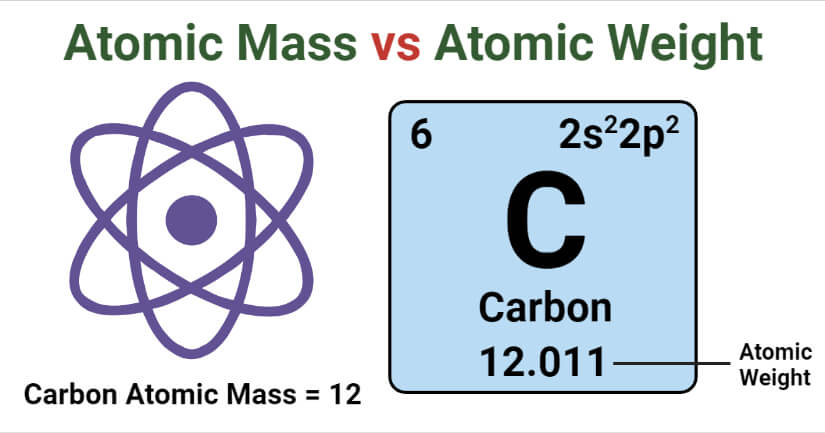
Atomic Mass Vs Atomic Weight Definition 7 Major Differences
Difference Between Atomic Number And Mass Number Definition Explanation With Examples
Difference Between Atomic Number And Atomic Weight Definition Explanation Difference
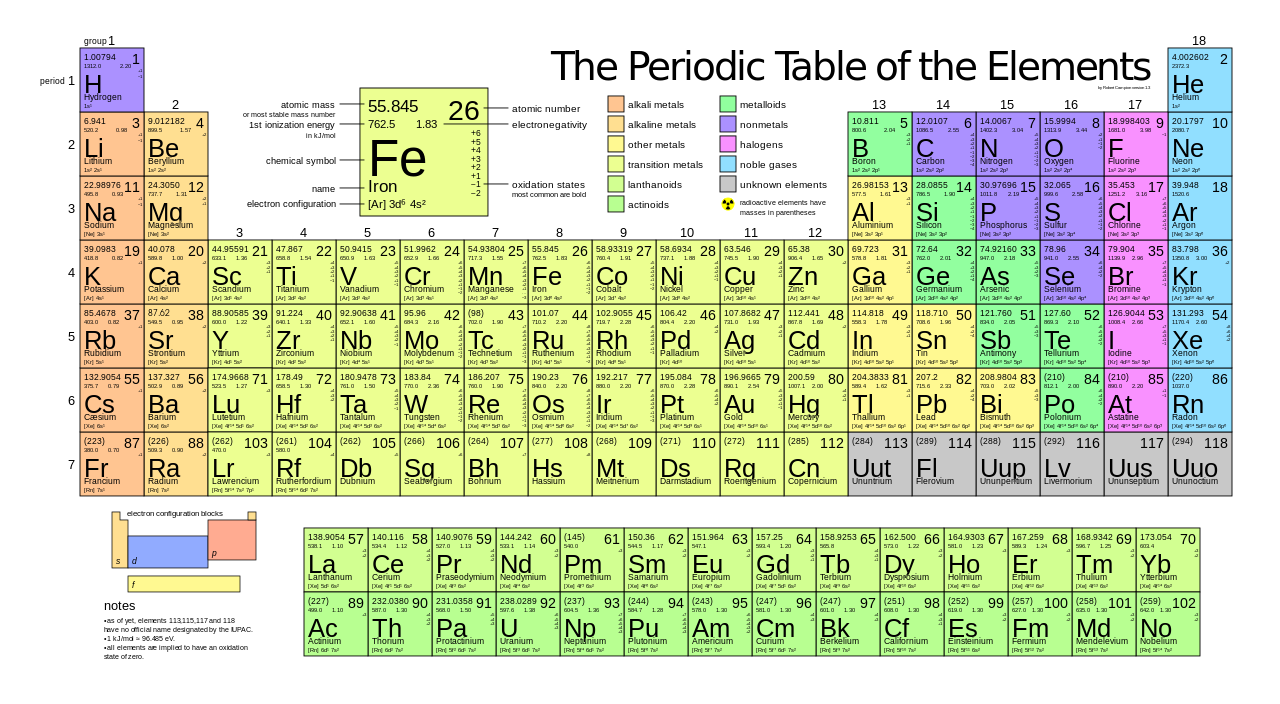
Difference Between Mass Number And Atomic Mass
Atoms Isotopes Ions And Molecules Boundless Biology
Difference Between Mass Number And Atomic Mass
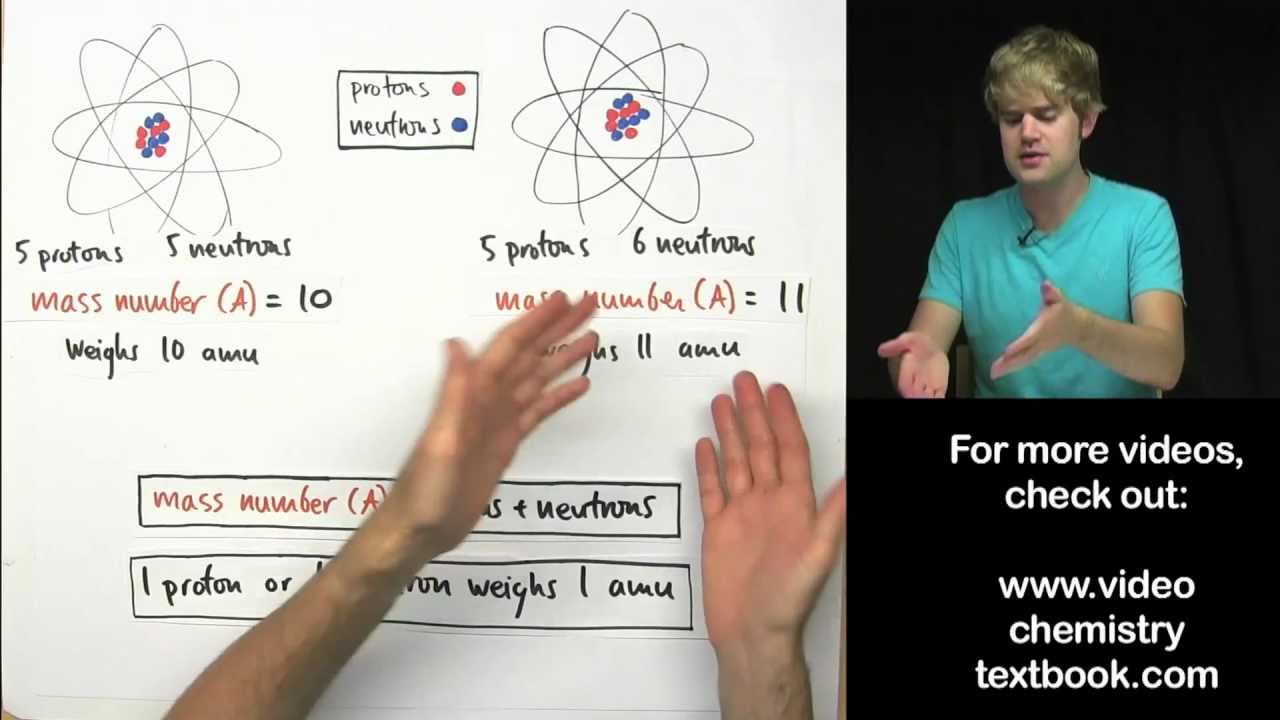
What S The Difference Between Mass Number And Atomic Mass Youtube

What Is An Atomic Number Definition And Examples

What S The Difference Between Mass Number And Atomic Weight Youtube

Atomic Number Atomic Mass And Isotopes Article Khan Academy
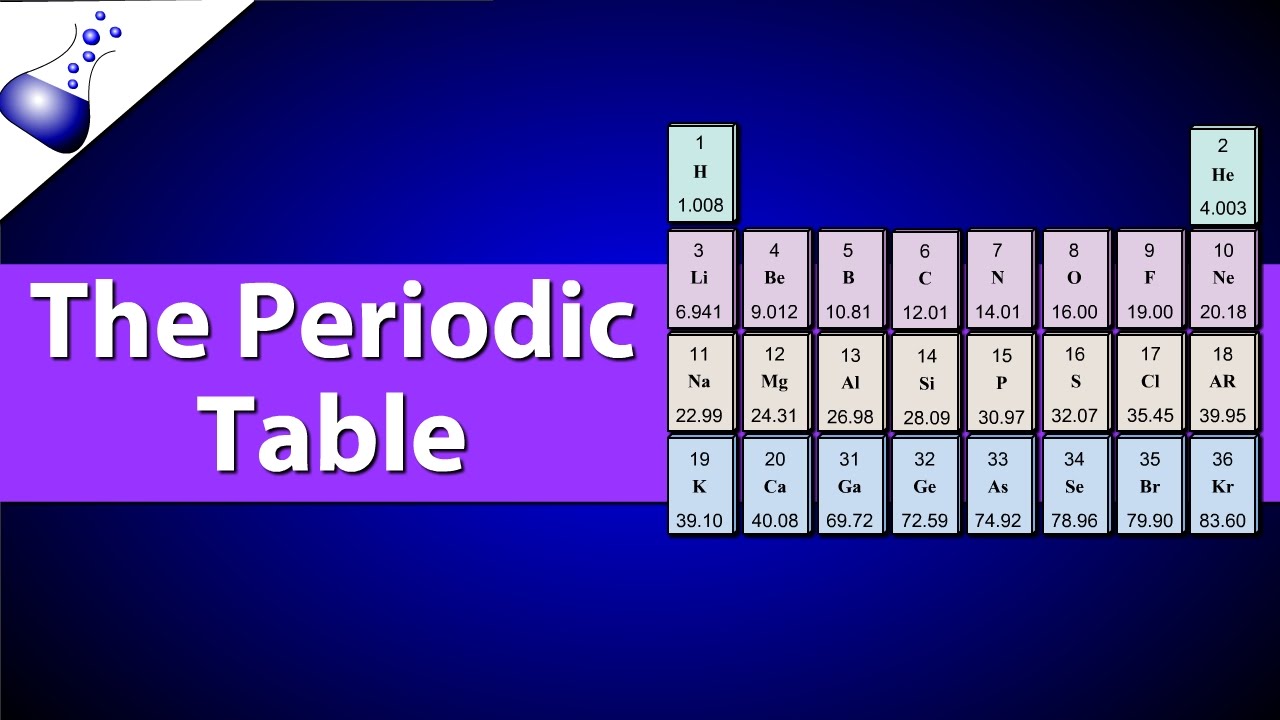
Understanding Atomic Number And Atomic Mass Youtube
Difference Between Atomic Number And Mass Number Definition Explanation With Examples

Average Atomic Mass Video Khan Academy
Atomic Number Atomic Mass And Isotopes Article Khan Academy

What Is An Atomic Number Definition And Examples

Atomic Number Mass Number How To Find The Atomic Mass Number Video Lesson Transcript Study Com
Comments
Post a Comment